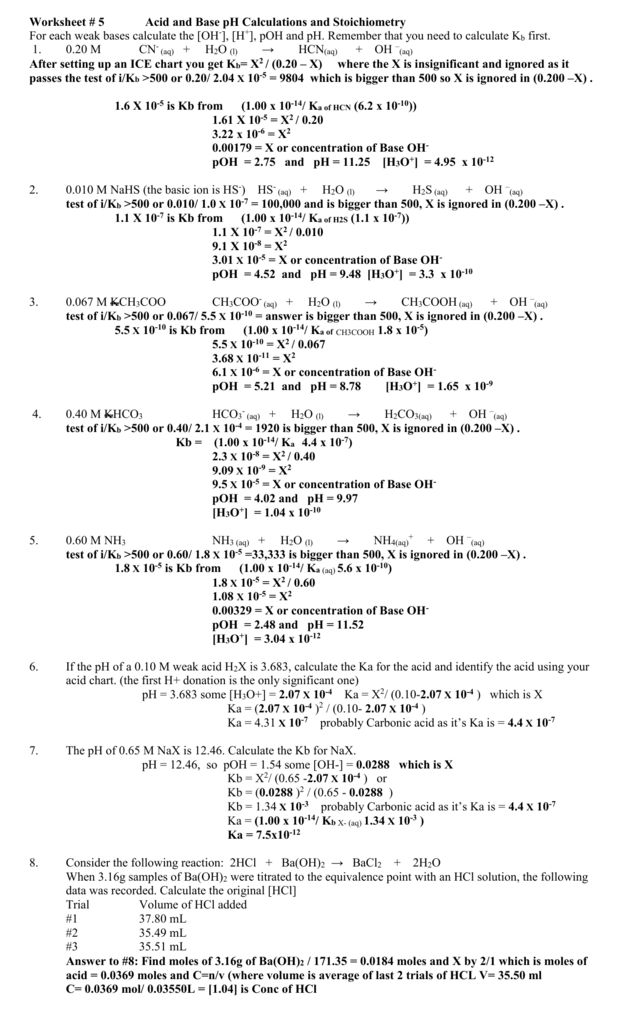
Worksheet 5 Acid and Base pH Calculations and Stoichiometry
This resource features three different versions of a worksheet on acids and bases: scaffolded, partially scaffolded and unscaffolded. Use the worksheets to identify learners' knowledge gaps and misconceptions once you have taught this part of the curriculum. Find out more about how to use this resource or download the worksheets below.
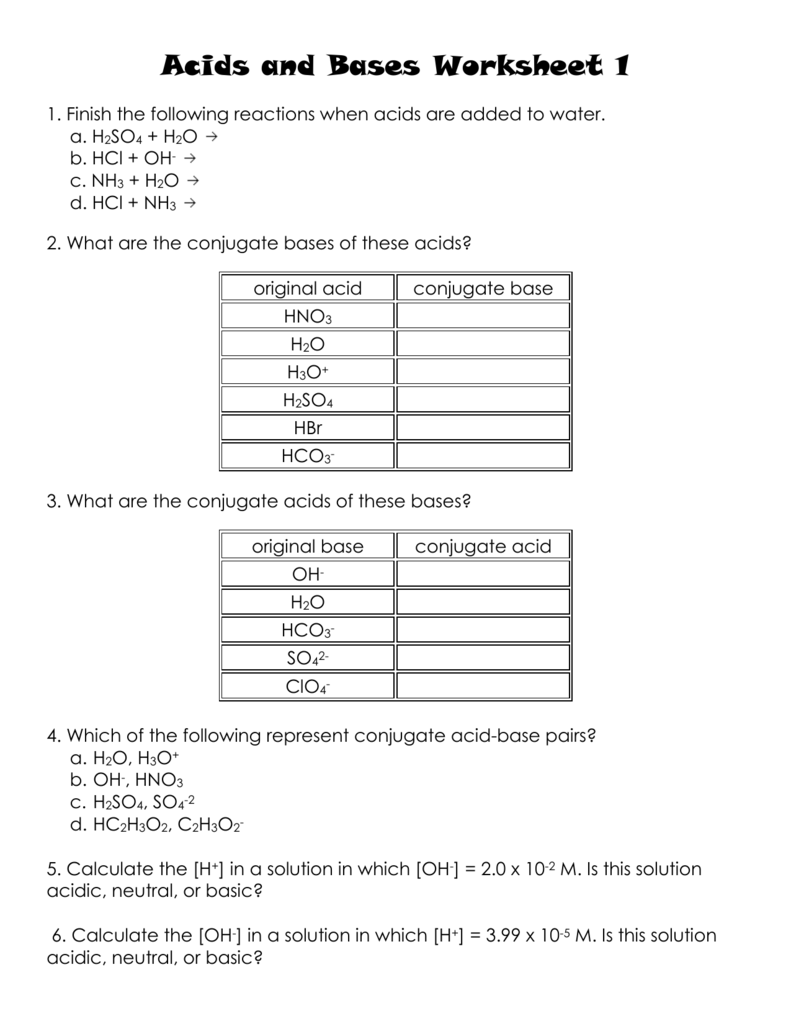
Acids and Bases Worksheet 1
Acids & Bases Calculations Practice Worksheet Directions: Solve the following pH calculations. Write the formula, plug numbers into formula, & give answer with. [OH-] pOH Acid / Base -1 x 10 3 M - 1 x 10 -8 M 6 2 -2.3 x 10 10 M -8.5 x 10 1 M -6.9 x 10 4 M -5.1 x 10 11 M . Author: Hughes, Brooke Created Date: 4/22/2015 2:25:31 PM.
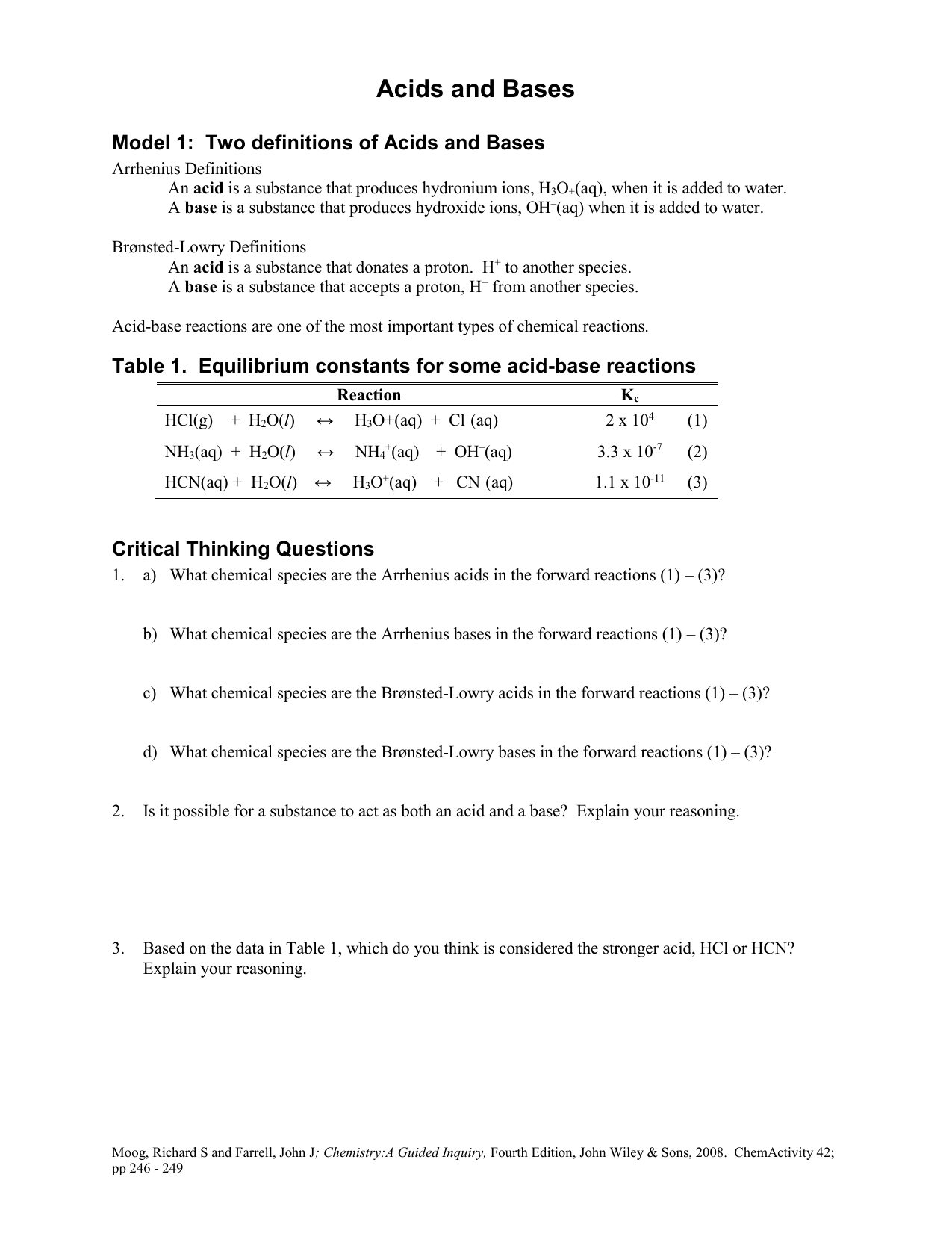
Acid Base Reaction Worksheet
Acid Base - pH Calculations Name: Date: Period: Seat #: Show all work strong acid solution - determine [H+], calculate pH (2.903) Calculate the pH of 0.00125M HNO. WORKSHEET #1 . diprotic acid solution - assume all [H+] from first ionization, determine [H+] using ICE box, calculate pH. Calculate the pH of 0.00125M H 2CO 3 K

Acids And Bases Calculations Practice Worksheet Answer Key
We have seen that the calculation of [H 3 O +] and pH for solutions of strong acids and base.To carry out a calculation of all species present in a solution of a pure weak acid in water requires use of the equilibrium constant for the acid's hydrolysis, called K a.In similar fashion, calculating the concentrations of all species in a solution of a weak base in water requires solving the.

Acids And Bases Worksheet
Acid and Base pH Calculations - Supplemental Worksheet KEY For each of the following solutions: Write a chemical equation, identify the limiting reactant (if there is one), and calculate the pH. We will calculate the pH of the solutions using the following 3 steps for each problem. Step 1: What is left in solution?

Acid and Base Worksheet 1 0708 ans key
Brønsted-Lowry. The Brønsted-Lowry definition of acids and bases liberates the acid-base concept from its limitation to aqueous solutions, as well as the requirement that bases contain the hydroxyl group. A Brønsted-Lowry acid is a hydrogen-containing species which is capable of acting as a proton (hydrogen ion) donor. A Brønsted-Lowry base is a species which is capable of acting.

Acids, Bases, and the pH Scale Worksheet Printable and Distance
Browse acid and bases calculations worksheet resources on Teachers Pay Teachers, a marketplace trusted by millions of teachers for original educational resources. Browse Catalog Grade Level Pre-K - K 1 - 2 3 - 5 6 - 8 9 - 12 Other Subject Arts & Music English Language Arts World Language Math Science Social Studies - History Specialty

Acids And Bases Calculations Practice Worksheet Answer Key
TASK 1 - Bronsted-Lowry acids & bases Identify the Bronsted-Lowry acid and base in each of the following reactions. SECTION 2 - pH of strong acids Number of protons released Monoprotic acid = acid that releases one H+ ion per molecule e.g. HCl (hydrochloric acid), HNO3 (nitric acid), CH3COOH (ethanoic acid) Diprotic acid =

Phet Acids And Bases Worksheet Phet Acid Base Worksheets Teaching
Introductory Chemistry (CK-12) 21: Acids and Bases
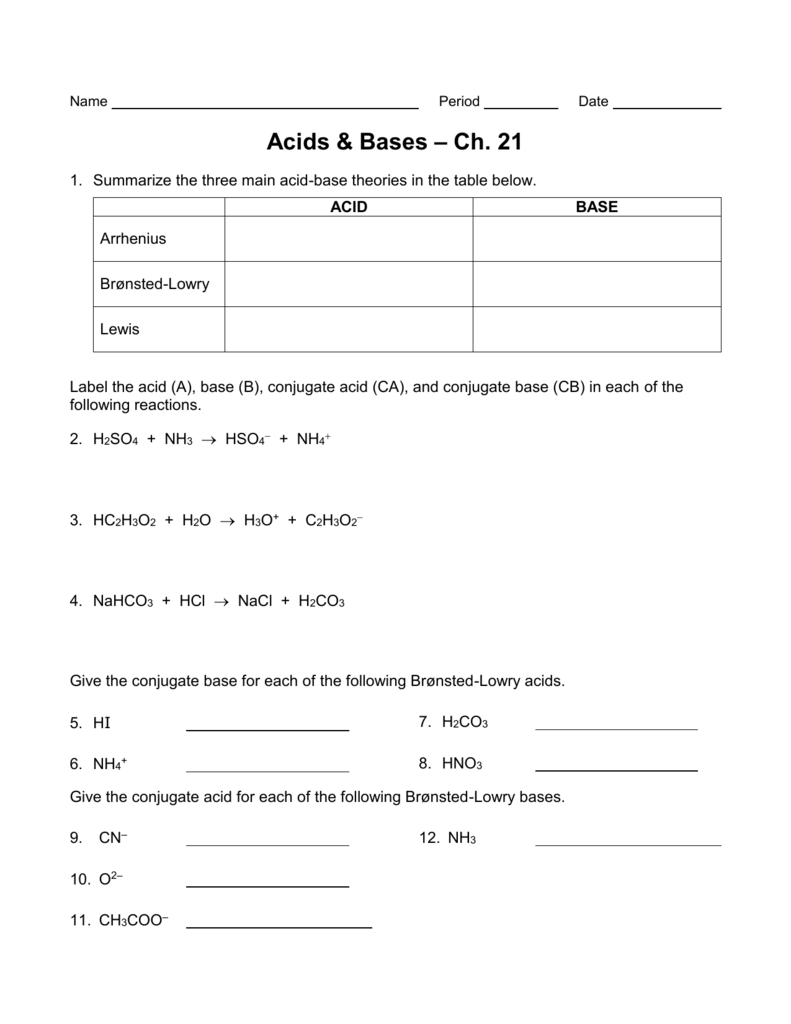
Acid And Base Worksheet
Given the solutions with the same initial concentration of each base, the higher percent of ionization is base A because it is the weakest base. 3. a. pKa + pKb = 14 → pKa = 14 − pKb = 14 − 3.80 = 10.2. Ka = 10 − pKa = 10 − 10.2 = 6.31 × 10 − 11. b. pKa + pKb = 14 → pKa = 14 − pKb = 14 − 7.90 = 6.10.

AcidBase Info and Practice Problems
Conceptual Questions. Acids, Bases, and Conjugates, Miscellaneous 1. In the Brønsted-Lowry definition of acids and bases, an acid __________ a. is a proton donor. b. is a proton acceptor. c. forms stable hydrogen bonds. d. breaks stable hydrogen bonds. e. corrodes metals. 2. In the Brønsted-Lowry definition of acids and bases, a base __________

Acids And Bases Worksheet
33% Download now of 2 Name pate Acids & Bases Calculations Practice Worksheet Directions: Solve the following pH calewlations. Write the formula, plug numbers ino formula, & give answer with 'corveet units and significant figures. 1. the pl a slain i 10.3, what isthe H5] oncntation? CH 2 tog"!

Conjugate Acid Base Pairs Worksheet PDF Acid Chemistry
In a strong acid - strong base titration, neutralization produces water and an aqueous solution of a salt, whose cation and anion come from the base and acid, respectively. Neither ion is acidic or basic, so the pH at the equivalence point is that of neutral water; i.e., 7.00.

Teach your students about the pH scale and calculating pH of acids and
1. For all the reactants and products, draw Lewis structures. 2. Identify the nucleophile (base) and electrophile (acid) in the reaction. 3. Draw curved arrows to show the flow of electrons. 4. Determine if the reaction can be termed a Brønsted-Lowry acid-base reaction. 7 PRACTICE PROBLEM
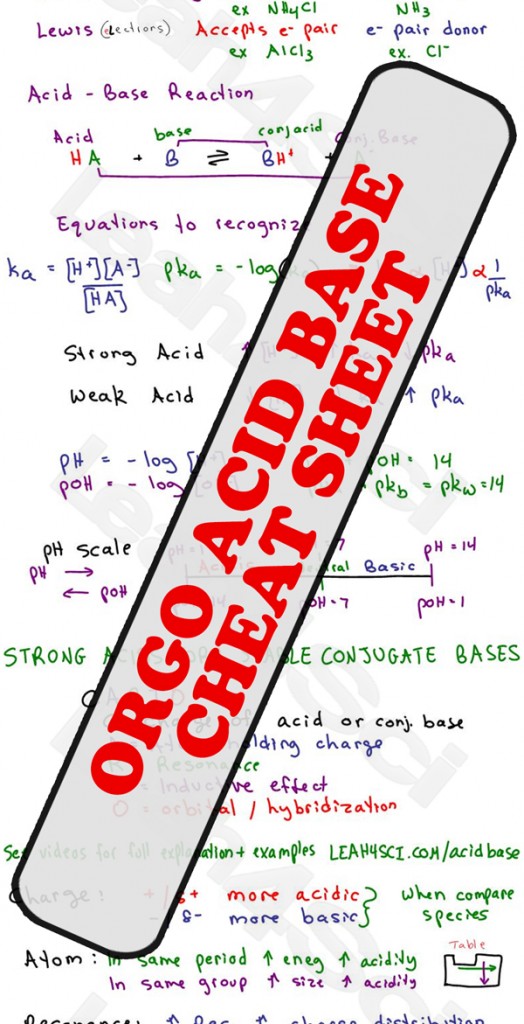
Acid Base pH and pKa Calculations in MCAT Chemistry Tutorial Video Series
Explain with equations and calculations, when necessary, whether an aqueous solution of each of these salts is acidic, basic, or neutral: \(KBr\) \(NH_4I\) \(KCN\) This page titled Acids and Bases (Worksheet) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Mark Draganjac via source content that was edited.
Acid and Base Worksheet Made By Teachers
ACIDS & BASES PRACTICE WORKSHEET 1. Without doing any calculations, identify each of the following solutions as acids or basic: a) [H 3 O +] = 3.4x10-8 M _____ b) [OH-. Identify the Bronsted-Lowry acid and base conjugate pairs for each of the following